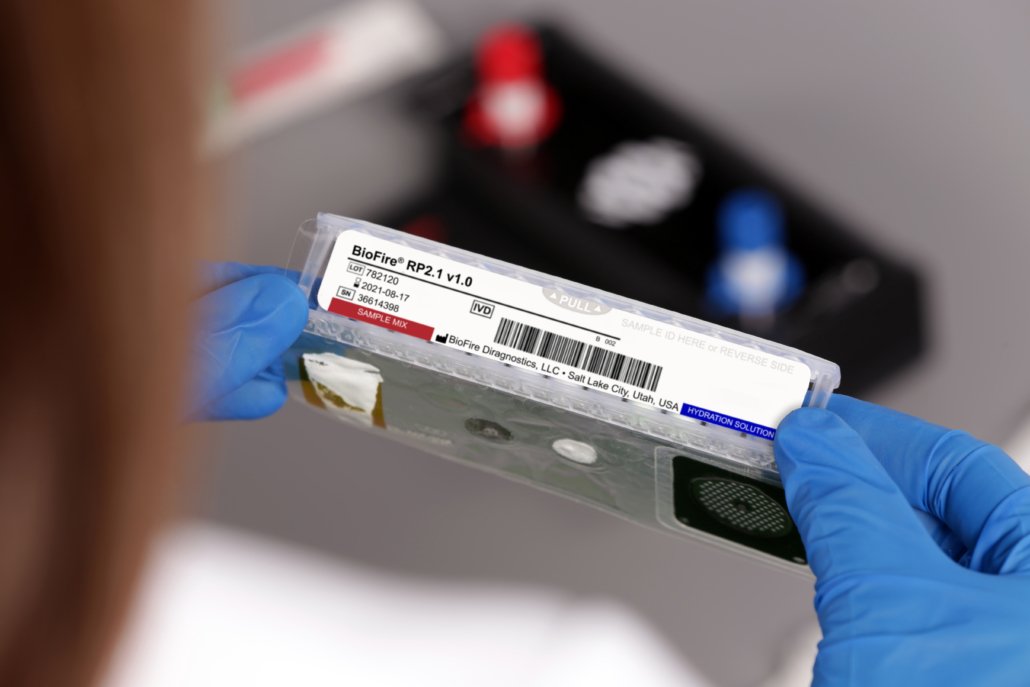
The BioFire® Respiratory 2.1
(RP2.1) Panel
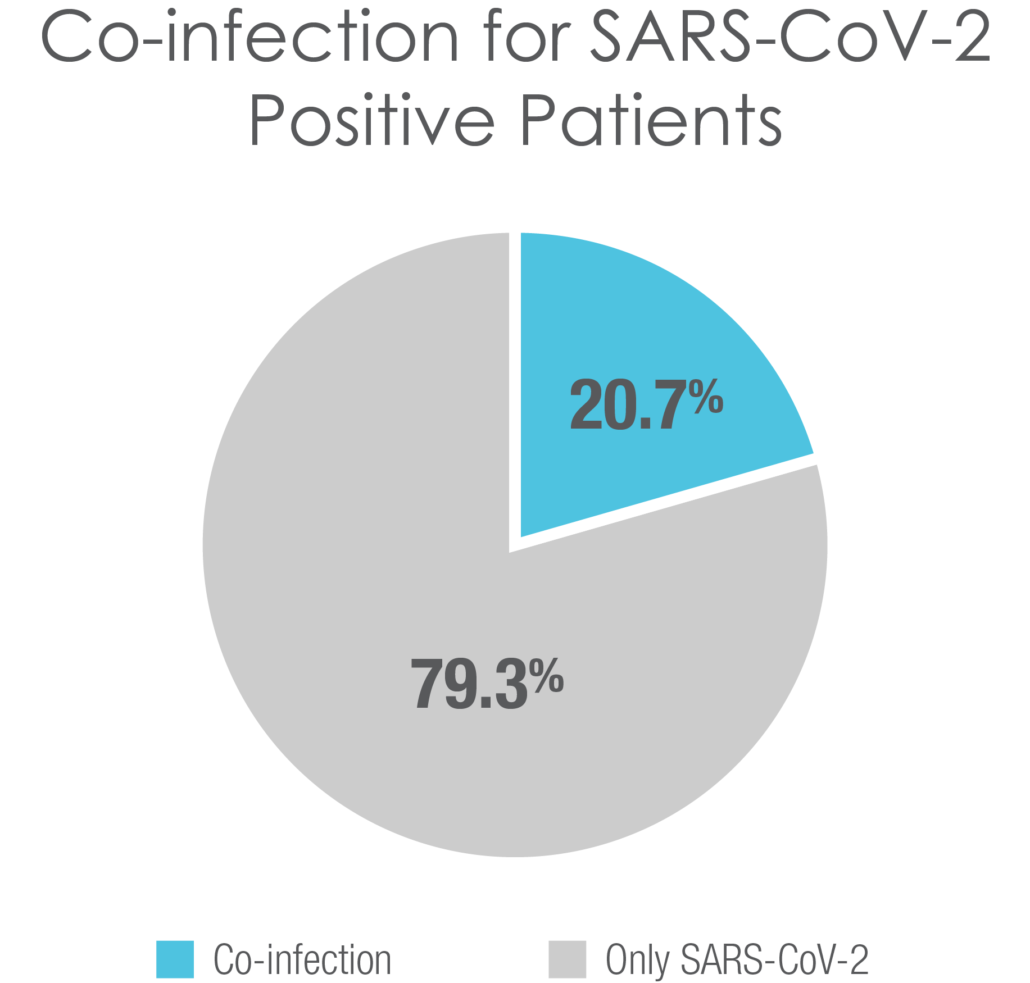
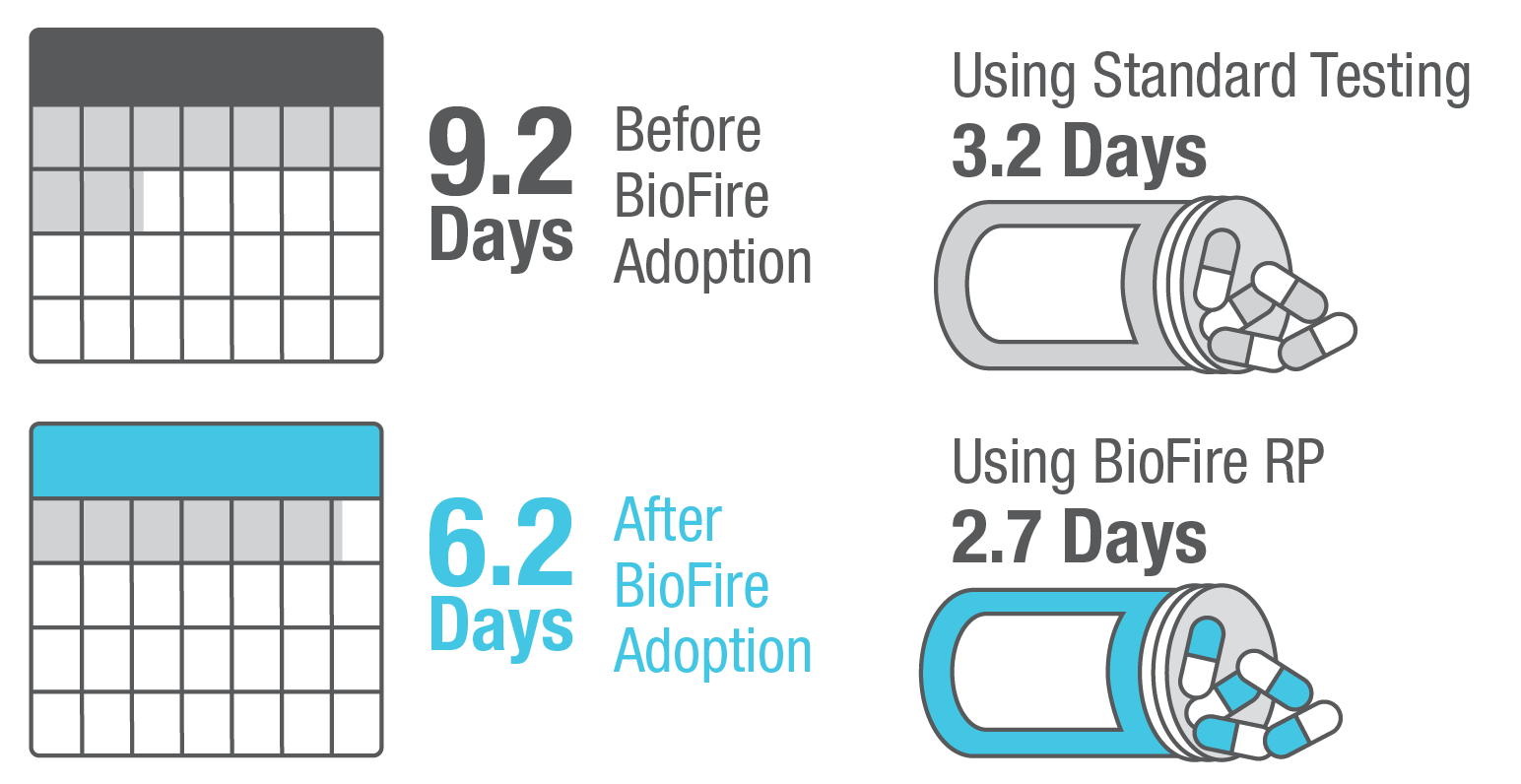
SARS-CoV-2 is a top concern for patients and clinicians, but several respiratory pathogens can cause nearly indistinguishable symptoms. The FDA De Novo authorized BioFire RP2.1 Panel is a frontline test to help clinicians quickly diagnose respiratory infections, including COVID-19, influenza, RSV, and many others.
Click here for information on BioFire’s additional COVID-19 solutions.