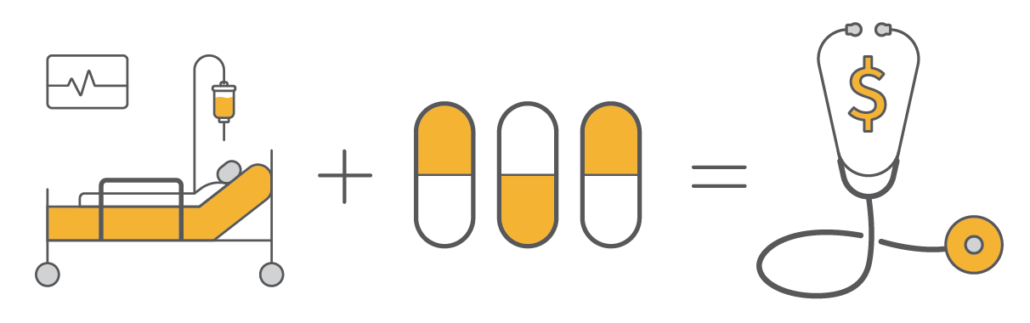
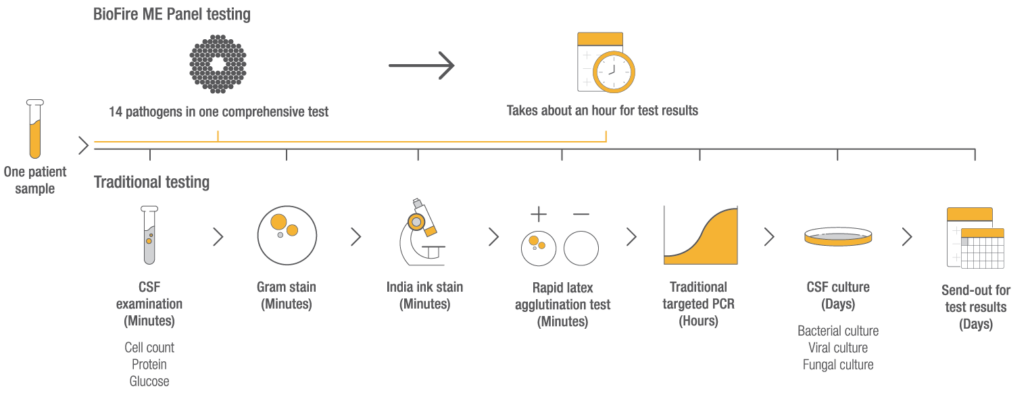
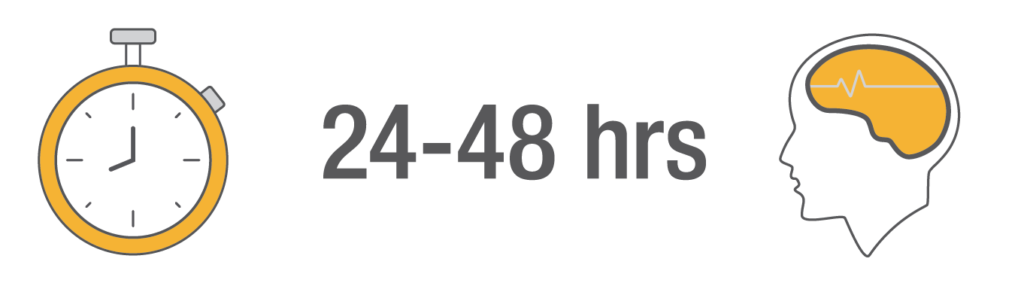Product availability varies by country. Consult your bioMérieux representative.
References
- Data on file, BioFire Diagnostics. The stated performance is the overall aggregate performance of the prospective clinical study data presented in the IFU.
- Confederation of Meningitis Organisations Fact Sheet. comomeningitis.org/facts. 2020.
- Bamberger D. Diagnosis, Initial Management, and Prevention of Meningitis. American Family Physician 2010 Dec 15;82:1491-1498.
- O’Brien M, et al. Impact of cerebrospinal fluid multiplex assay on diagnosis and outcomes of central nervous system infections in children: a before and after cohort study. The Pediatric Infectious Disease Journal 2018;37:868-71.
- Evans M, et al. Impact of the implementation of a rapid meningitis/encephalitis multiplex polymerase chain reaction panel on IV acyclovir duration: multicenter, retrospective cohort of adult and pediatric patients. Diagnostic Microbiology and Infectious Disease 2020;96(2):114935.
- Posnakoglou L, et al. Impact of cerebrospinal fluid syndromic testing in the management of children with suspected central nervous system infection. European Journal of Clinical Microbiology & Infectious Diseases Jul 2020.
- Cailleaux M, et al. Impact of a multiplex PCR assay (FilmArray®) on the management of patients with suspected central nervous system infections. European Journal of Clinical Microbiology & Infectious Diseases 2019;39(2):293-297.
- Moffa M, et al. Impact of a Multiplex Polymerase Chain Reaction Assay on the Clinical Management of Adults Undergoing a Lumbar Puncture for Suspected Community-Onset Central Nervous System Infections. Antibiotics (Basel) 2020;9(6):282.
- Hagen, A., Eichinger, A., Meryer-Buehn, M., Schober, T., & Huebner, J. (2020). Comparison of antibiotic and acyclovir usage before and after the implementation of an on-site FilmArray meningitis/encephalitis panel in an academic tertiary pediatric hospital: a retrospective observational study. BMC Pediatrics, 20(56). https://doi.org/10.1186/s12887-020-1944-2
- Lyons T, et al. Impact of in-hospital enteroviral polymerase chain reaction testing on the clinical management of children with meningitis. Journal of Hospital Medicine 2012;7:517-520.
Guidelines
A. Tunkel A, et al. Practice Guidelines for the Management of Bacterial Meningitis. Clin Infect Dis 2004;39:1267-1284.
B. Tunkel A, et al. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2008;47:303-327.