What Went Into SPOTFIRE
When developing the SPOTFIRE Respiratory Solution, bioMérieux had three things in mind:
- Making an instrument easy enough to use in a CLIA-waived setting
- Bringing PCR-based testing to the point of care
- Delivering syndromic results in a clinically relevant time frame
From the onset, we knew we had to bring the syndromic approach to point-of-care settings. And given that the pandemic highlighted the need for better POC respiratory testing, we turned to the BIOFIRE® Respiratory 2.1 (RP2.1) Panel.
With a target menu of 22 probable pathogens, including SARS-CoV-2, the BIOFIRE R2.1 Panel was a game changer during the pandemic. The associated risk with COVID-19 made differential respiratory diagnoses increasingly important, as they helped clinicians determine the appropriate quarantine and treatment measures.
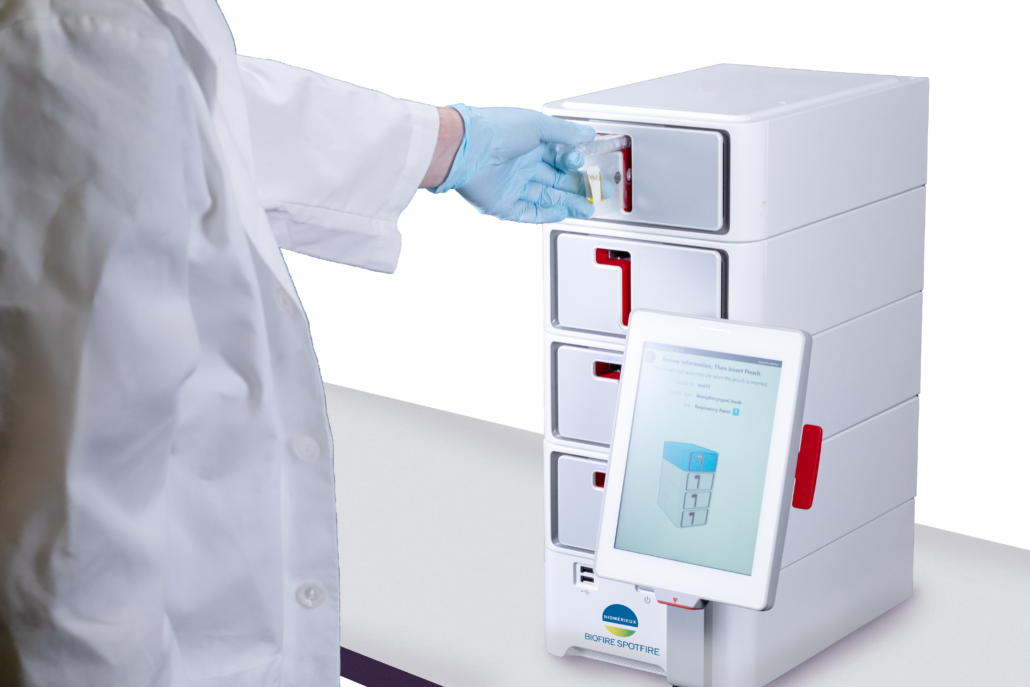
However, the BIOFIRE RP2.1 Panel was created for a laboratory setting, and we needed to bring that same quality of testing to the CLIA-waived market. In that vein, we tailored the SPOTFIRE System’s panel menus to include the most common pathogens that cause respiratory tract infections. We also optimized the chemistry to run faster and perform better. And with that the expanded SPOTFIRE R Panel was born. One PCR test of 15 targets—including 11 viruses and four bacteria—can produce results in about 15 minutes. The targeted SPOTFIRE R Panel Mini, developed alongside the SPOTFIRE R Panel, tests for 5 viral targets in the same time frame, bringing new possibilities and options to clinicians.
Another game-changing element is the user-friendly nature of the SPOTFIRE System interface. Keeping testing in-house may streamline the diagnostic workflow and get answers to patients during their clinic visit, which is why the SPOTFIRE System is designed specifically to be used by non-laboratory professionals.