Molecular Testing
Molecular tests developed for diagnostic use can either detect targeted genetic material—like nucleic acid—or identify specific protein signatures—like MALDI-ToF. A nucleic acid amplification test (NAAT) is a type of molecular test that may use polymerase chain reaction (PCR) (or similar technologies such as NASBA, deep sequencing, etc.) to amplify a specific sequence of nucleic acid. Catalyzed by rapid temperature cycling, PCR is a chemical reaction that exponentially amplifies the targeted nucleic acid to detectable levels. One cycle of PCR doubles the amount of genetic material in the starting sample. Running multiple cycles of PCR produces millions to billions of copies. A molecular PCR test can detect targeted nucleic acid even if it is only present in small amounts in the patient sample.
NAATs have a wide variety of useful applications in laboratory medicine, including infectious disease diagnostics, oncology, and genetics. For example, an oncologist could use molecular testing of nucleic acid to help tailor cancer treatment based on the specific genetic abnormalities detected in a patient’s tumor. Similarly, an infectious disease physician could use a NAAT to rapidly determine the pathogenic cause of an infection and identify genetic markers that may indicate antibiotic resistance, helping them optimize treatment as well. A molecular test can also detect chromosomal abnormalities or changes that may elevate a patient’s risk of developing a particular disease or disorder.
When it comes to infectious disease diagnostics, a NAAT can provide laboratories with critical advances in accuracy and speed compared to traditional standard of care methods, such as culture. In turn, laboratories that use molecular testing solutions can help healthcare providers get the answers they need sooner to optimize treatment.
Syndromic Molecular Testing for Infectious Disease Diagnostics
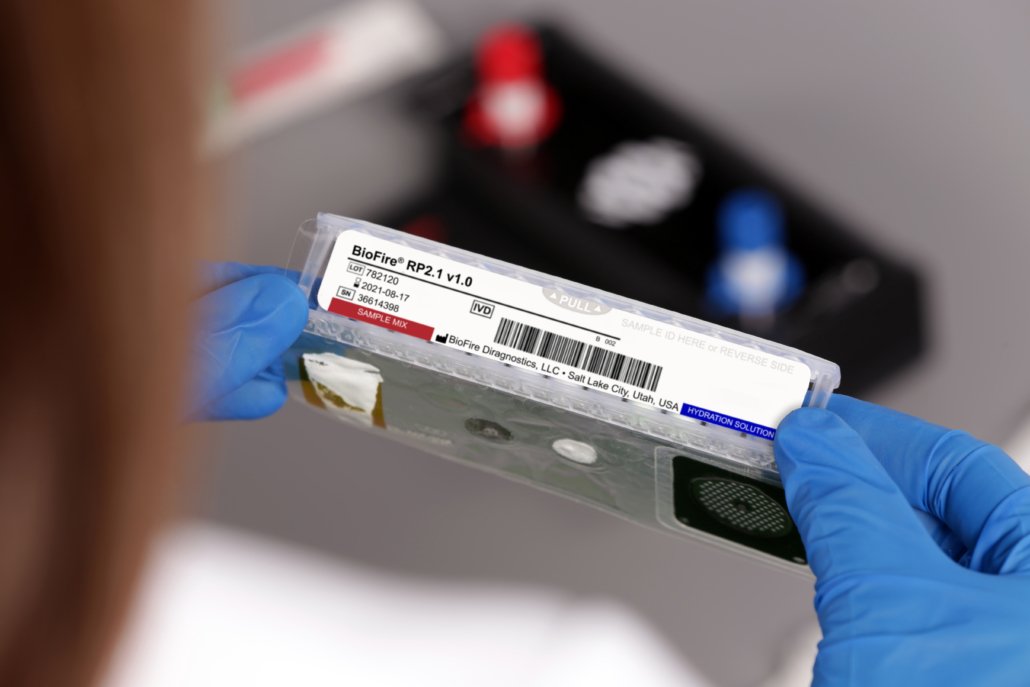
Syndromic testing is a symptom-driven, molecular approach to infectious disease diagnostics. The BioFire® FilmArray® System uses multiplex PCR (a type of PCR in which multiple PCR reactions are combined and performed simultaneously) to perform syndromic tests. Each multiplex panel on the BioFire System targets a comprehensive grouping of pathogens associated with a particular syndrome. Some BioFire® FilmArray® Panels also target relevant antimicrobial resistance genes.
BioFire offers syndromic testing solutions to streamline and simplify the process of infectious disease diagnostics for hospitals, laboratories, and clinics. By detecting targets at the molecular level—including bacteria, viruses, fungi, parasites, and antimicrobial resistance markers—the BioFire System can be more sensitive than culture and more efficient than a serial testing approach.
Traditional testing often involves growing the organism in culture, which can be difficult and time-consuming and requires the organism in the patient sample to be viable. Many viral infections do not have reliable diagnostic tests. Additionally, many commonly used tests don’t target all the potential causes of a patient’s infection, which leads to downstream testing and invasive procedures.
Molecular Testing for SARS-CoV-2 Detection
The ongoing COVID-19 pandemic prompted the creation of molecular diagnostic solutions that could quickly and accurately detect the SARS-CoV-2 virus, including the BioFire® Respiratory 2.1 (RP2.1) Panel with FDA De Novo authorization and the BioFire® Respiratory 2.1 EZ (RP2.1-EZ) Panel (EUA).* A PCR test for COVID-19 typically offers higher sensitivity than a COVID-19 rapid antigen test, which directly detects the presence or absence of specific viral antigens present in a patient’s sample.1
Learn more about BioFire’s response to the COVID-19 pandemic and how syndromic testing with SARS-CoV-2 detection can impact your institution.
*This test has not been FDA cleared or approved; this test has been authorized by FDA under an EUA for use by authorized laboratories; this test has been authorized only for the detection and differentiation of nucleic acid of SARS-CoV-2 from multiple respiratory viral and bacterial organisms; and this test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics tests for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
Reference:
- https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html.