The BioFire® FilmArray® Blood Culture Identification 2 Panel
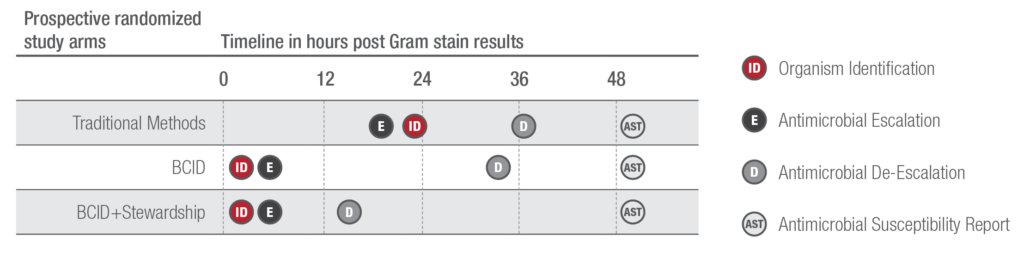
Early identification and treatment of sepsis are essential to combat one of the leading causes of hospital patient deaths.1 With 43 targets, the expanded and FDA-cleared BioFire® Blood Culture Identification 2 (BCID2) Panel detects pathogens and antimicrobial resistance genes directly from positive blood cultures.