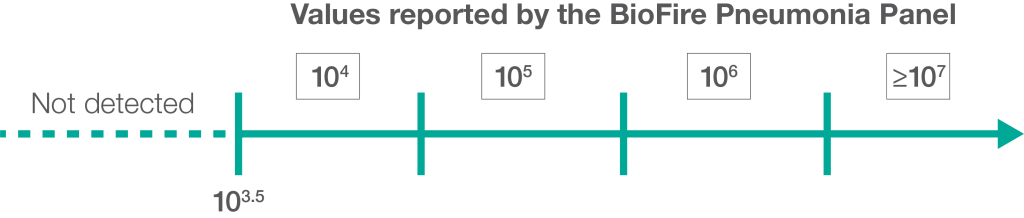
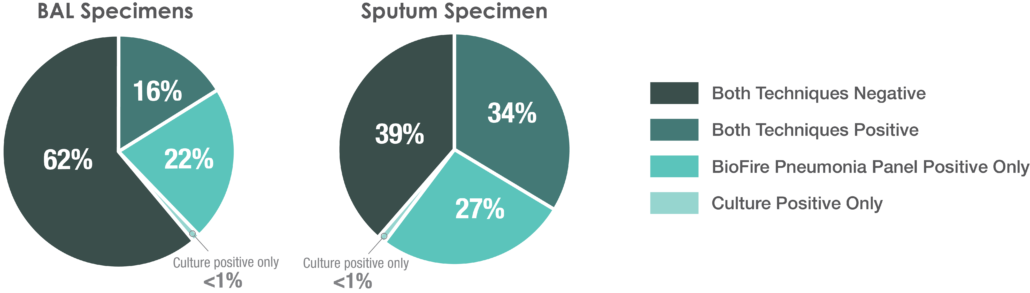
The BioFire® FilmArray®
Pneumonia (PN) Panel
Syndromic infectious disease testing for Pneumonia.
Pneumonia patients are frequently over-treated with antibiotics because it is difficult to quickly identify the pathogen responsible for their symptoms. The BioFire Pneumonia Panel delivers fast, accurate pathogen identification in about one hour, which may allow physicians to put patients on appropriate therapy quicker.1,2